57
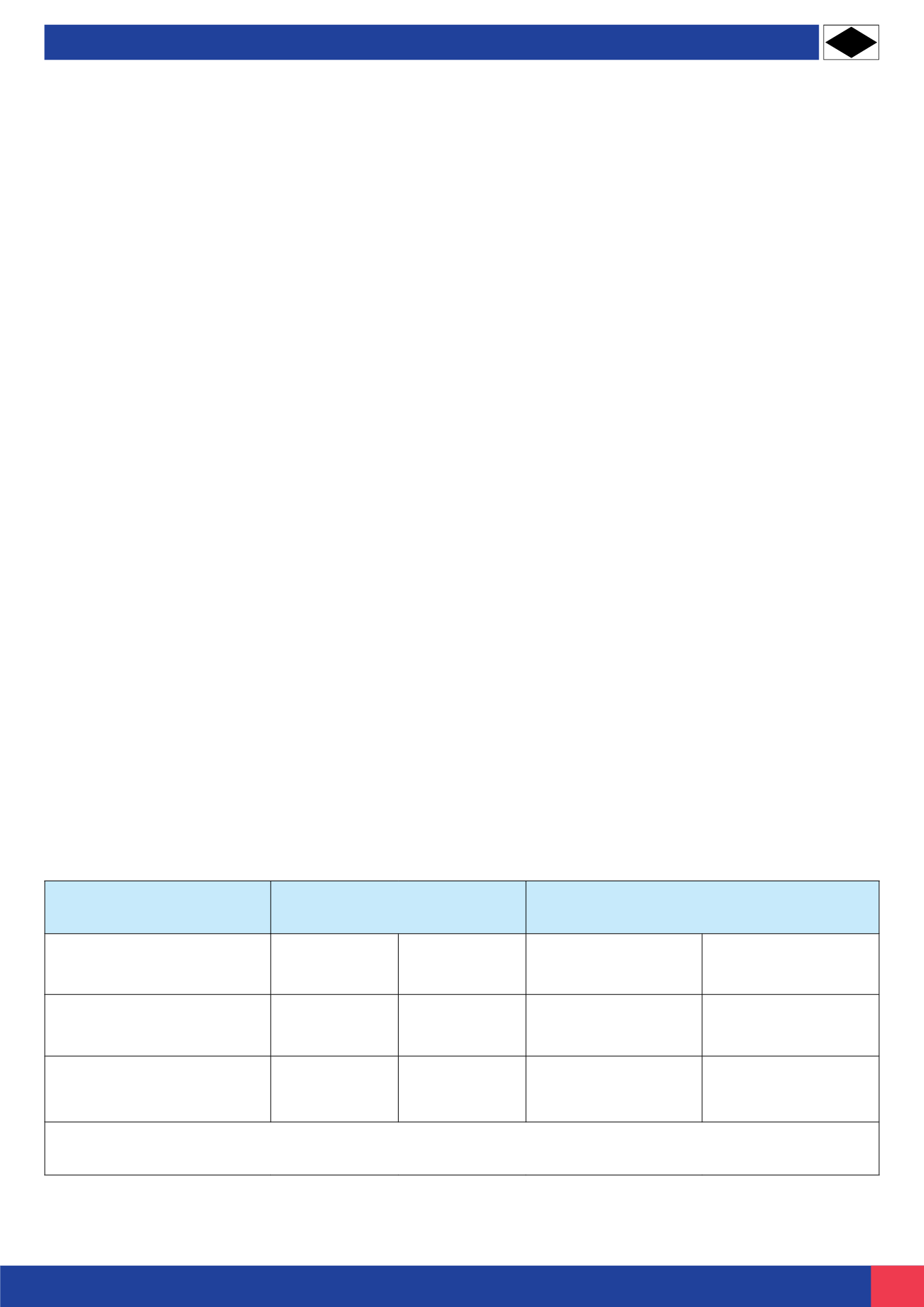
Building
Roof or wall washed by rain
Eaves, Soffits or sheltered
areas not washed by rain
Structure
No deposits
Deposits
accumulate
No deposits
Deposits
accumulate
Cleaning in rural, suburban and
residential areas
4/year
6/year
12/year
24/year
Cleaning for seaside,industrial
and severe areas
8/year
12/year
24/year
52/year
Notes:
Cleaning frequency is dependant on surface finish, design details, environment, cleaning procedure and expectations of performance.
Cleaning involves using Pro-Railing Enviro-Shield
(189900902)
- which comprises stainless steel cleaner, demineralised water and protection spray.
Degreasing
Dirt left on the surface after a fabrication process can
have a serious affect on the corrosion resistance of
stainless steel. Not only will it prevent the steel from
oxidizing, it can contain corrosive particles, which will
start rusting at a later date.
Pickling
Pickling requires the use of strong chemicals
(hydrofluoric acid and nitric acid) to dissolve the
surface of the steel. This process completely
removes any surface contaminants and will help to
restore the chromium level to the weld affected areas.
The heat from the welding process drives chromium
away from the weld area. The area adjacent to the
weld is often low in chromium and high in iron. These
areas are always the most susceptible to corrosion
once the component is in use.
Iron dissolves more readily than chromium, therefore,
the pickling process leaves the surface chromium-rich
and in a condition where it can form a dense oxide
layer.
Passivation
Stainless steel will passivate in the open atmosphere
- assuming that it is spotlessly clean to begin with
and so is the air it is sat in. It is a slow process and,
depending on the grade of stainless, can take
between 24-48 hours to occur.
Stainless steel is not self-cleaning!
The chromium-oxide layer that protects stainless
from corrosion is relatively fragile. It can be broken or
damaged during fabrication, if scratched and/or
surface contamination is allowed to settle on the
surface.
The most effective way to form the passive layer is
to force it by subjecting the steel to an oxidizing
chemical. These are typically acid solutions
containing nitric acid or citric acid. This process
speeds up the reaction time and typically takes
around 2-3 hours contact time to fully form a dense
and effective layer.
Simply cleaning a corroded piece of stainless steel
with a stainless steel cleaner and a scouring pad
(usually phosphoric acid or citric acid based) does
not passivate it. These acids will help to dissolve the
corrosion (iron oxides) and leave the surface in a
condition in which it can self-passivate.
Rain washing regularly will reduce the risk of tea
staining (brown discolouration). This is a visual
impairment only, and does not affect the structural
integrity or longevity of the material. The best way to
prevent it is to follow the cleaning chart below, but as
a general rule of thumb the recommendation is that
the stainless needs cleaning as often as the glass.
Remember bad design can result in poor
performance e.g. partially sheltered systems will
greatly reduce the benefit of natural washing by rain.
Maintenance of stainless steel should be considered
in the design process.
Recommended cleaning intervals for stainless steel handrail systems
CLEANING INTERVALS


